
Nina Shah MD, discusses the future of CAR T-cell therapy and bispecific antibodies in multiple myeloma.

Nina Shah MD, discusses the future of CAR T-cell therapy and bispecific antibodies in multiple myeloma.

Evidence-Based Oncology regulatory updates for August 2020 feature coverage of Kite Pharma's new CAR T-cell therapy and selinexor in DLBCL.

Evidence-Based Oncology managed care updates for August 2020 feature coverage of liquid biopsies, companion diagnostics, and CAR T-cell therapy.

A phase 2 study found that data from a 3-year follow up showed statistically significant improvements in overall survival for patients with high-risk locally advanced squamous cell carcinoma of the head and neck treated with an IAP antagonists with chemo-radiation therapy.
Debio 1143 in combination with standard cisplatin-based concomitant fractionation chemoradiation therapy was found to induce a statistically and clinically significant improvement in overall survival compared with CRT alone in patients with high-risk locally advanced squamous cell carcinoma of the head and neck.

Peter Martin, MD, provides an overview of what the current MCL treatment paradigm looks like as well as areas of future exploration and debate.

The FDA this week granted the fourth Orphan Drug Designation for a novel gene therapy product candidate (OCU400, Ocugen Inc.) in the treatment of PDE6B gene mutation-associated retinal diseases.
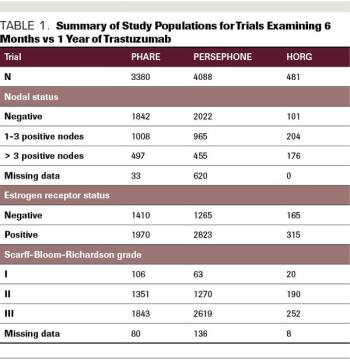
ABSTRACT Prior to the introduction of trastuzumab, the first targeted anti-HER2 agent, in 1998, patients diagnosed with HER2-positive breast cancer felt like they were being handed a death sentence. Despite treatment with aggressive chemotherapy, their tumors recurred faster, more often spread to brain and liver, and were associated with higher rates of death than HER2-negative tumors. However, in the 1980s, cancer researchers and oncologists recognized that HER2 could be targeted by a small molecule that binds to the receptor on the cell surface and blocks the signal telling the cell to divide. This small molecule was called trastuzumab, and it eventually completely changed how HER2-positive breast cancer was treated. The drug was first approved in the metastatic setting, and then the results of 2 pivotal randomized control trials demonstrated that the administration of trastuzumab in the adjuvant setting decreased the risk of breast cancer recurrence by 50%. These trials showed trastuzumab to be unequivocally effective in the adjuvant setting and the HERA trial results led to the adoption of 1 year of adjuvant trastuzumab as the standard of care. Since that time, the field of anti–HER2-targeted therapy has exploded, with the development of multiple targeted agents for use in the advanced and up-front settings. Although trastuzumab significantly improves outcomes for women diagnosed with HER2-positive breast cancer and has few adverse effects (AEs), the disadvantages are that it requires intravenous administration every 3 weeks and can be associated with cardiac AEs. It is also expensive. Given all of these factors, the question of whether a duration of trastuzumab that is shorter than 1 year may be acceptable for some patients with early-stage HER2-positive breast cancer is an important and very relevant one. Here, we will review the studies that have examined this question and evaluate their results.
Gene therapy to treat choroideremia shows potential for maintaining or improving vision

Sintilimab injection plus pemetrexed and platinum-based therapy led to a statistically significant improvement in progression-free survival compared with chemotherapy alone as a first-line treatment for patients with locally advanced or metastatic nonsquamous non��–small cell lung cancer.

A CD30-targeted CAR T-cell therapy was found to elicit a high rate of durable complete responses (CRs) in heavily pretreated patients with relapsed/refractory Hodgkin lymphoma.

Paul J. Shaughnessy, MD, discusses how CAR T-cell therapy is being utilized in hematologic malignancies, challenges faced with this modality, and ongoing research efforts being made to better leverage its use.
Intravitreal injection offers safety, efficacy, and improved vision in patients

Investigators from John Theurer Cancer Center participated in the pivotal ZUMA-2 clinical trial, which assessed the safety and effectiveness of brexucabtagene autoleucel in patients with relapsed or refractory mantle cell lymphoma.

Bristol Myers Squibb and bluebird bio submitted a second Biologics License Application (BLA) to the FDA for idecabtagene vicleucel (ide-cel), the companies’ investigational B-cell maturation antigen–directed chimeric antigen receptor (CAR) T-cell immunotherapy, indicated for adults with relapsed and refractory multiple myeloma (MM).

The FDA granted breakthrough therapy designation to osimertinib for the adjuvant treatment of patients with early-stage epidermal growth factor receptor-mutated non-small cell lung cancer after complete tumor resection with curative intent.

The FDA has granted a breakthrough therapy designation to osimertinib for the adjuvant treatment of patients with stage IB, II, and IIIA EGFR-mutated non–small cell lung cancer following complete resection with curative intent.

The FDA has granted a breakthrough therapy designation to MK-6482 for the treatment of patients with von Hippel-Lindau disease–associated renal cell carcinoma who have nonmetastatic tumors of less than 3 centimeters, unless immediate surgery is necessitated.

The FDA granted breakthrough therapy designation to MK-6482 for the treatment of patients with von Hippel-Lindau disease-associated renal cell carcinoma and orphan drug designation to MK-6482 for von Hippel-Lindau disease.

No serious adverse events reported through 24 weeks of a 52 week trial.

The introduction of tyrosine kinase inhibitor therapy earlier in the treatment timeline for patients with EGFR-mutant non–small cell lung cancer holds the promise of improving overall survival for this patient population.
The European Medicines Agency has granted access to the PRIME initiative for the Specific Peptide Enhanced Affinity Receptor T-cell therapy ADP-A2M4 for the treatment of patients with synovial sarcoma.

Assessing the value of novel therapies has been challenging and controversial using existing methods, pointing to the need for continued exploration of new approaches.

New phase 1/2 data shows AAV-RGPR may benefit a previously untreated population of inherited retina disease boys and young men.
The therapy, to be sold as Tecartus, will be used to treat adult patients with mantle cell lymphoma who have relapsed or not responded to other treatments.

The agent is now the first and only approved CAR T-cell therapy to treat adult patients with relapsed or refractory mantle cell lymphoma.

The FDA has approved the CAR T-cell therapy brexucabtagene autoleucel (Tecartus; formerly KTE-X19) as a treatment for adult patients with relapsed/refractory mantle cell lymphoma.

Umbilical cord blood provides a readily available source of stem cells to provide better access to hematopoietic stem cell transplantation to safely and effectively treat various noncancerous genetic disorders in children.

During ASCO, Janssen presented results from the CHRYSALIS study on amivantimab, a bispecific antibody being developed to treat non–small cell lung cancer (NSCLC). The pharma giant, along with its parent company, Johnson & Johnson (J&J), had previously received a breakthrough therapy designation in December for teclistamab, another bispecific antibody indicated for potential treatment of multiple myeloma.

The FDA has cleared an investigational new drug application for the first-of-its-kind, off-the-shelf CAR T-cell product FT819, which targets CD19-positive malignancies.