This study found that found that mini-strokes and heart attacks were significantly less common among patients with locally advanced non-small cell lung cancer who underwent proton therapy versus conventional photon-based radiation therapy.

This study found that found that mini-strokes and heart attacks were significantly less common among patients with locally advanced non-small cell lung cancer who underwent proton therapy versus conventional photon-based radiation therapy.

The study presented at AAO provides an update on previously shared 6-month data from earlier this year.

Chimeric antigen receptor (CAR) T cells can be highly effective, but the durability of the therapy has been lacking in many patients with hematological malignancies. Many efforts are underway to fix this problem.
New research suggests it’s not disruptive to give radiation therapy to patients who are awaiting CAR T-cell therapy for relapsed or refractory multiple myeloma.
Adagrasib, a novel agent aimed at KRAS G12C mutations, has demonstrated early signs of efficacy in patients with advanced non–small cell lung cancer and colorectal cancer whose tumors harbor the alteration, raising hopes for a new therapy against a challenging oncogenic target.
D. Ross Camidge, MD, PhD discusses the role of targeted therapy and how it is rapidly expanding in the treatment paradigm of patients with non–small cell lung cancer.

The emergence of cellular-based therapies represents a major opportunity to improve outcomes in the heavily pretreated and refractory myeloma population.

As academic medical centers gain knowledge of how to administer CAR T-cell therapy and manage adverse events, these institutions can be a bridge to bring therapies to community practice.

The combination of lenvatinib plus pembrolizumab demonstrated encouraging antitumor activity in patients with metastatic clear cell renal cell carcinoma who had progressed on prior PD-1 or PD-L1 immune checkpoint inhibitor therapy.

A panel of lymphoma experts discuss several novel agents for relapsed/ refractory diffuse large B-cell lymphoma, some of which have been recently approved.
The FDA has lifted the clinical hold placed on the phase 1 PSMA-101-001 study of the CAR T-cell therapy P-PSMA-101 in patients with metastatic castration-resistant prostate cancer.

The vice president for Clinical Services at OneOncology describes challenges and opportunities for chimeric antigen receptor (CAR) T-cell therapy in the community practice setting.

Faiz Anwer, MD, discusses the growing role of CAR T-cell therapy in multiple myeloma and areas of unmet need.

Abhinav Deol, MD, highlights the progress that has been made with CAR T-cell therapy in leukemia and lymphoma, challenges faced with regard to accessibility and toxicity, and next steps for this modality.
In a longitudinal cohort study, researchers identified 17 seemingly novel variants of the PDE6A retinitis pigmentosa gene, suggesting it may be amenable to gene therapy.

The investigational wholly-owned allogeneic CAR T-cell therapy CTX110 demonstrated dose-dependent efficacy and responses in patients with relapsed/refractory CD19-positive B-cell malignancies.
Single-agent daratumumab as maintenance therapy improved progression-free survival compared with observation in patients with newly diagnosed multiple myeloma eligible for autologous stem cell transplant.

Brian T. Hill, MD, PhD, reflects on how the approval of CAR T-cell therapy has impacted clinical practice for his patients with MCL, ongoing research with practice-changing implications, and how MCL treatment has evolved during the coronavirus disease 2019 pandemic.
Intravitreal gene therapy continues to be well tolerated and shows robust efficacy

Brian T. Hill, MD, PhD, discusses the advent of CAR T-cell therapies, such as axicabtagene ciloleucel, tisagenlecleucel, and brexucabtagene autoleucel, and how they have shifted lymphoma treatment into a new era.

The European Medicines Agency’s Committee for Medicinal Products for Human Use has adopted a positive opinion for KTE-X19 as a treatment in adult patients with relapsed/refractory mantle cell lymphoma who previously received 2 or more lines of systemic therapy, including a BTK inhibitor.
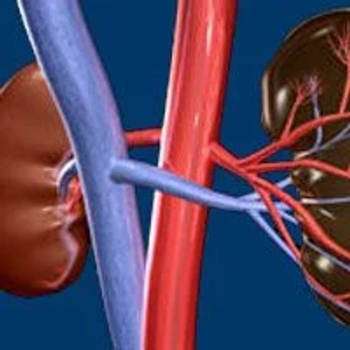
Targeted therapy and immunotherapy have improved survival outcomes for patients with metastatic renal cell carcinoma.

New data showing efficacy and tolerability with ADP-A2AFP, alpha-fetoprotein–directed specific peptide enhanced affinity receptor T-cells, suggest that further research evaluating the 5 billion or more cell dosing regimen is warranted in hepatocellular carcinoma.

Patients treated with Axovant’s gene therapy AXO-Lenti-PD reported improvements of 40% in UPDRS-III scores, and 71% improvement in activities of daily living.

The process of approving a new therapy for relapsing and/or remitting mantle cell lymphoma therapy got off to a faster start in China, but United States regulators caught up and approved the drug first.
Researchers identified molecular and cellular characteristics of anti-CD19 CAR T-cell infusion products associated with how patients with large B-cell lymphoma respond to treatment and experience adverse events.

The investigational advanced cell therapy omidubicel resulted in rapid platelet engraftment and reduced the number of infections and hospitalizations in patients with high-risk hematologic malignancies, meeting all 3 secondary end points of a phase 3 trial.

The FDA has granted a breakthrough therapy designation to IMGN632 for the treatment of patients with relapsed or refractory blastic plasmacytoid dendritic cell neoplasm.

The FDA granted breakthrough therapy designation to IMGN632 for the treatment of patients with relapsed or refractory blastic plasymacytoid dendritic cell neoplasm.

Solid Biosciences noted that its response to the agency requests for further information about manufacturing as well as updated safety and efficacy data on all dosed patients led to the lift.