
Cytokine release syndrome represents a major concern, and source of costs, associated with the life-saving gene therapy.

Cytokine release syndrome represents a major concern, and source of costs, associated with the life-saving gene therapy.

Though chimeric antigen receptor (CAR) T-cell therapy has been largely touted as one of the most important advances in cancer care in recent years, the therapy comes with the risk of severe toxicities as well as increased financial burden due to the high cost of the drugs.
A recent prospective phase 2 study sought to investigate autologous hematopoietic stem cell transplantation as a therapeutic intervention in multiple sclerosis (MS).
A recent report from the National Comprehensive Cancer Network (NCCN) investigated the current state of chimeric antigen receptor (CAR) T-cell therapy and future strategies to consider as the novel immunotherapy evolves and is used in the treatment of more patients.
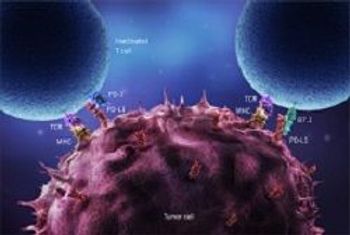
Only 1 day after the European Commission approved CAR T-cell therapies, the National Institute for Health and Care Excellence deemed the treatment too expensive to justify on Britain's state-funded health service.
Researchers from the University of Texas MD Anderson Cancer Center, along with the Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI), recently published guidelines in Nature Reviews Clinical Oncology for the management of chimeric antigen receptor (CAR) T-cell therapy for children with acute lymphoblastic leukemia.

Celyad, a biopharmaceutical company that focuses on the development of chimeric antigen receptor (CAR) T-cell therapies, recently announced that the FDA has accepted its Investigational New Drug (IND) application for CYAD-101, the first non–gene-edited allogeneic clinical program.
Drug manufacturer AbbVie, and a nonprofit drug discovery division of Scripps Research, Calibr, announced earlier this week that they are partnering to develop chimeric antigen receptor (CAR-T) cell therapies primarily aimed at treating cancer, particularly, solid tumors.
Last week, Bristol-Myers Squibb announced that the China National Drug Administration approved the country’s first immuno-oncology and first PD-1 therapy, nivolumab (Opdivo), for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC).
Last year, the FDA expanded the indications of rheumatoid arthritis (RA) drug tocilizumab (Actemra) to include the treatment of cytokine release syndrome (CRS) caused by CAR T-cell therapy. Recently, 2 studies have identified another rheumatoid arthritis drug that could be more effective in the treatment of CRS.

A study recently published in Cell investigated the deletion of the CD33 protein to enable CAR T-cells to more accurately target and attack cancerous cells in patients with n acute myeloid leukemia (AML).
A new case study found that an acute myeloid leukemia patient has remained cancer free for 9 months following treatment with the chimeric antigen receptor (CAR) T-cell treatment, CYAD-01, and a bone marrow transplant.
Published: January 5th 2019 | Updated:
Published: June 15th 2018 | Updated:
Published: June 20th 2018 | Updated:

Published: July 16th 2019 | Updated:
Published: June 29th 2018 | Updated:

Published: June 5th 2019 | Updated: