
NY-ESO-1 SPEAR T-cell therapy showed promising results and was reasonably safe in patients with synovial sarcoma, according to a new study. The addition of fludarabine may be important to achieving those positive results.

NY-ESO-1 SPEAR T-cell therapy showed promising results and was reasonably safe in patients with synovial sarcoma, according to a new study. The addition of fludarabine may be important to achieving those positive results.

The FDA has approved brentuximab vedotin (Adcetris) for the treatment of primary cutaneous anaplastic large-cell lymphoma and CD30-expressing mycosis fungoides in patients who have received prior systemic therapy.

A variety of adoptive T-cell therapy strategies have shown promise in clinical studies with recent FDA approvals granted to CAR-modified T-cell therapies, representing the potential for future combination strategies.

The FDA has approved brentuximab vedotin (Adcetris) as a treatment for patients with cutaneous T-cell lymphoma who have received prior systemic therapy.

The FDA approved alectinib as first-line therapy for ALK-positive non-small cell lung cancer based on results of the ALEX study, which showed significant improved PFS compared with crizotinib.

The FDA today lifted clinical holds placed on 2 phase I trials investigating a gene-edited allogeneic CAR T-cell therapy known as UCART123.
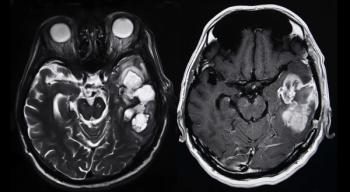
More than 25% of patients with recurrent, high-grade glioma treated with a gene therapy combination were alive more than 3 years after treatment, according to data from a subset of patients in a phase I clinical trial.

Novartis has submitted an application to the FDA to extend the indications for its CAR T-cell therapy tisagenlecleucel (Kymriah) for its use in adult patients with relapsed or refractory diffuse large B-cell lymphoma who are not eligible for autologous stem cell transplant.

Alfred L. Garfall, MD, MS, discussed the treatment and management of patients with multiple myeloma, with a focus on maintenance therapy, CAR T-cell therapy, and the role of transplant.

Edward A. Stadtmauer, MD, sheds light on the future of chimeric antigen receptor T-cell therapy, systemic therapeutic advances in the field of acute myeloid leukemia, and remaining challenges in the multiple myeloma paradigm.

David G. Maloney, MD, PhD, discusses the progress made with CAR T cells, as well as the challenges that still exist in the use of this therapy.

The FDA has granted accelerated approval to acalabrutinib (Calquence) for the treatment of mantle cell lymphoma in adult patients who have received at least one previous therapy.

The FDA has granted an accelerated approval to acalabrutinib as a treatment for adult patients with mantle cell lymphoma following at least 1 prior therapy.

A team of investigators from multiple institutions has proposed new guidelines for monitoring, grading, and managing the side effects of CAR T-cell therapy.

Reem Karmali, MD, discusses ongoing key trials of CAR T-cell therapy, the chronic safety concerns with the treatment, and what combinations have the most potential.

Andre Goy, MD, discusses the recent success with CAR T-cell therapy, and what is on the horizon for this therapeutic option across hematologic malignancies.

CD19 CAR T cells are highly effective in high-risk patients with chronic lymphocytic leukemia who have failed to respond to treatment with ibrutinib, a Bruton's tyrosine kinase inhibitor, according to a new study.

Axicabtagene ciloleucel (Yescarta) has been approved to treat adult patients with diffuse large B-cell lymphoma who have not responded to or who have relapsed after at least 2 other kinds of treatment.

The FDA has approved the CAR T-cell therapy axicabtagene ciloleucel (Yescarta) for the treatment of large B-cell lymphomas in adults who have failed or relapsed after two or more prior treatments.

The FDA has approved the CD19-directed CAR T-cell therapy axicabtagene ciloleucel as a treatment for adults with relapsed or refractory non-Hodgkin lymphoma.

Maintenance therapy with lenalidomide following autologous stem-cell transplantation conferred a survival benefit for patients with newly diagnosed multiple myeloma.


Evidence-Based OncologyTM sat down with Brandon R. Shank, PharmD, MPH, BCOP, clinical pharmacy specialist, Division of Pharmacy, The University of Texas MD Anderson Cancer Center, to understand a pharmacist's role in administering chimeric antigen receptor (CAR) T cells.

An advisory committee gave unanimous support to Luxturna, 3 months prior to its BLA ruling.

The second-generation EGFR tyrosine kinase inhibitor dacomitinib significantly improved progression-free survival over gefitinib as a first-line therapy for EGFR–positive non–small-cell lung cancer, according to a randomized phase III trial.

Defined composition CAR T cells directed against CD19 have potent anti-tumor activity in B cell malignancies, including acute lymphocytic leukemia.

The FDA has awarded Breakthrough Therapy Designation to osimertinib for first-line treatment of patients with metastatic EGFR mutation-positive non-small cell lung cancer (NSCLC).

A new therapy has succeeded in restoring leg function in mouse models, though the researchers say it will be years before such a therapy can be tried in humans.

Adjuvant therapy with tyrosine kinase inhibitors for patients with high-risk renal cell carcinoma (RCC) who have undergone a nephrectomy may be supported by level IIa evidence from the National Comprehensive Cancer Network guidelines.

Chimeric antigen receptor T-cell therapy for hematological malignancies took a huge step forward this summer with the FDA approval of Novartis