
The CAR T-Cell therapy MB-102 has been granted an Orphan Drug Designation by the FDA, according to Mustang Bio, Inc, the manufacturer of the investigational treatment.

The CAR T-Cell therapy MB-102 has been granted an Orphan Drug Designation by the FDA, according to Mustang Bio, Inc, the manufacturer of the investigational treatment.

Michael Wang, MD, provides insight on recent advances in mantle cell lymphoma as well as research efforts for patients with a high risk of relapse.

Cytokine release syndrome represents a major concern, and source of costs, associated with the life-saving gene therapy.
A new study looked at the rates of response and remission in patients with relapsed or refractory follicular lymphoma who underwent CD19-directed CAR-T cell therapy.

The CAR T-cell therapy lisocabtagene maraleucel elicited a 71% overall response rate as well as a tolerable safety profile in a cohort of patients with relapsed/refractory mantle cell lymphoma.
A new study looked at targeted vs nontargeted therapy in real world patients with renal cell carcinoma.

A phase 1/2 clinical trial is expected to be initiated in the second half of 2019.

Investigators see potential in adding targeted therapy to dual immunotherapy for intermediate- and poor-risk patients with renal cell carcinoma. In the phase III COSMIC-313 trial, investigators aim to evaluate cabozantinib, nivolumab, and ipilimumab in patients with untreated advanced RCC.

Alison R. Sehgal, MD, discusses the latest developments with CAR T-cell therapy in lymphomas.

Preclinical trials and success stories suggest that much is riding on vector-based therapies for the treatment of rare neurological conditions.

Mazyar Shadman, MD, MPH, discusses earlier use of CAR T-cell therapy in lymphoma, the impact of approved products on future development, and recent data with chemotherapy-free and time-limited therapy in chronic lymphocytic leukemia.

PF-06939926 is a recombinant adenoassociated virus serotype 9 (AAV9) capsid carrying mini-dystrophin, a shortened version of the human dystrophin gene, under the control of a muscle-specific promoter.

Researchers conducted a cost-effectiveness analysis on the two FDA-approved CAR T-cell therapies for diffuse large B-cell lymphoma.

Patients with high-risk relapsed/refractory chronic lymphocytic leukemia who failed or were intolerant of ibrutinib derived more benefit from CD19‐targeted CAR T-cell therapy when the BTK inhibitor was concurrently administered than when it was not.

The CAR T-cell therapy lisocabtagene maraleucel demonstrated high rates of response, including minimum residual disease in blood and marrow in patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma.
Lurbinectedin may represent a new treatment option as second-line therapy for patients with small-cell lung cancer.
Researchers tested radiation therapy as a bridging therapy for patients with relapsed/refractory diffuse large B-cell lymphoma during the interval between T-cell collection and final CAR T administration.

Abeona Therapeutics is currently planning a phase 1/2 clinical trial to evaluate ABO-202 in Batten disease.

Reem Karmali, MD, MS, shares early data with ibrutinib maintenance therapy and highlights recent advances and challenges in the treatment of patients with mantle cell lymphoma.

The FDA has granted an accelerated approval to single-agent pembrolizumab for the treatment of patients with metastatic small cell lung cancer who have disease progression on or after platinum-based chemotherapy and ≥1 other prior line of therapy.

Axovant reported positive safety data as well as improvements in a number of measurements of motor function and dyskinesias in patients with Parkinson disease who received treatment with their investigational gene therapy.

Ahmed Galal, MD, sheds light on the current use of CAR T-cell therapy in relapsed/refractory lymphomas and a handful of strategies to expand the reach of this therapy.

Though chimeric antigen receptor (CAR) T-cell therapy has been largely touted as one of the most important advances in cancer care in recent years, the therapy comes with the risk of severe toxicities as well as increased financial burden due to the high cost of the drugs.
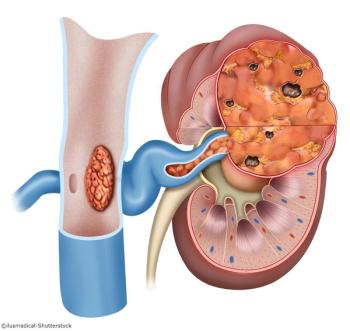
A pilot study evaluated cytoreductive surgery combined with immune checkpoint therapy in patients with metastatic renal cell carcinoma.
A phase I study of a CAR T-cell therapy showed success in refining cell dosing and adverse event management protocols in patients with relapsed/refractory ALL.

Every week, The American Journal of Managed Care® recaps the top managed care news of the week, and you can now listen to it on our podcast, Managed Care Cast.

Ahead of the ASCO Annual Meeting, we discuss the assessment and management of cytokine release syndrome in patients with cancer with Elizabeth Shpall, MD.

BioMarin announced the investigative gene therapy reached pre-specified criteria for Factor VIII levels in adult patients with severe hemophilia A.

AveXis—a Novartis company—announced that it will work with payers to implement 5-year outcomes-based agreements and novel pay-over-time options. The company also said it will offer a patient program to support affordability and access.

This is the first gene therapy approved for a devastating condition that leads to permanent ventilation or death for many patients by age 2.