
Zolgensma, an adeno-associated virus vector-based, one-time gene therapy administered via intravenous infusion, is the first and only FDA-approved gene therapy for SMA.

Zolgensma, an adeno-associated virus vector-based, one-time gene therapy administered via intravenous infusion, is the first and only FDA-approved gene therapy for SMA.

Previous analyses into MultiStem Cell Therapy have shown benefits and safety in stroke patients. What will it take for the therapy to reach a phase 3 trial for ARDS?

Early-stage study results from the multi-center MUST-ARDS trial show the ex-vivo adult progenitor cell-expanding therapy is capable of improving 28-day mortality, care burden, and overall safety in patients with ARDS.
A new study identified several T-cell antigens that are shared between tumor tissue and skin in a cohort of patients with non–small-cell lung cancer who were treated with anti–PD-1 therapy.
Insights about where stem cell treatment of neurological diseases is headed.

New early-stage data suggest that vector‐mediated gene silencing of striatal CaV1.3 expression may hold promise for preventing the induction of levodopa-induced dyskinesias in Parkinson disease.
Voxelotor, a first-in-class oral therapy, is both safe and effective in sickle cell disease, according to a phase 1/2 randomized study assessing the drug. These findings were consistent across all doses, ranging from 500 to 1000 mg.

The FDA has granted an orphan drug designation to the autologous CAR T-cell therapy P-BCMA-101 for the treatment of patients with relapsed/refractory multiple myeloma.
This review summarizes the current evidence supporting the use of SBRT as treatment for inoperable renal cell carcinoma, as well as provides recommendations for patient selection and reviews the technical aspects of treatment and the expected toxicities.

Two novel CAR T-cell therapies designed to attack solid tumors are showing signs of antitumor activity and tolerability in early clinical trial findings, fueling optimism about expanding this emerging form of immunotherapy beyond hematologic malignancies.

Nirav N. Shah, MD, discusses the promise of CAR T-cell therapy in hematologic malignancies and highlights recent research exploring sequential CAR-T therapies, armored CAR T cells, as well as other novel approaches.

In a pair of phase 2 trials and a phase 1 study, patients with SMA types 1 and 2 treated with the gene therapy displayed a number of motor milestone achievements and a prolonged event-free survival rate.

Healthcare is very comfortable with treating a disease, but it’s not as good with handling cures. However, the advent of gene therapy and precision medicine means more and more expensive cures are coming down the pipeline, said panelists on the last day of Asembia’s 15th annual Specialty Pharmacy Summit, held April 29 to May 2 in Las Vegas, Nevada.
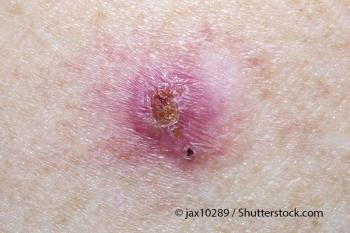
Researchers evaluated the response of patients with BCC to fractionated photodynamic therapy at 30 days after treatment.
Researchers tested the addition of the T cell–boosting decitabine to anti–PD-1 therapy with camrelizumab among patients with relapsed or refractory classic Hodgkin lymphoma.

Christine N. Duncan, MD, discusses the current components of CAR T-cell therapy in pediatric acute lymphoblastic leukemia.
Study results demonstrated that in adult patients with blastic plasmacytoid dendritic cell neoplasm, the targeted therapy resulted in high response rates, particularly among treatment-naïve patients.

The combination of lenalidomide (Revlimid) with rituximab (Rituxan), cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP; R2-CHOP) did not improve progression-free survival compared with placebo and R-CHOP as a frontline therapy in patients with activated B-cell-type diffuse large B-cell lymphoma, missing the primary endpoint of the phase III ROBUST trial.

Tagraxofusp led to a 90% overall response rate as a first-line therapy in patients with blastic plasmacytoid dendritic cell neoplasm.
Modifications to the engineering of the chimeric antigen receptor (CAR) mean that the patient produces fewer cytokines and has time to clear them before they build up in the bloodstream.
As part of CMS’ FY 2020 Medicare Hospital Inpatient Prospective Payment System and Long-Term Acute Care Hospital Prospective Payment System Proposed Rule and Request for Information, the agency is proposing an increase in how much it reimburses hospitals for chimeric antigen receptor (CAR) T-cell therapy, as well as wage index hikes for rural hospitals.

Omar Nadeem, MD, highlights exciting approaches that have emerged in relapsed/refractory myeloma, such as CAR T-cell therapy, and shares unanswered questions that future research should address.
Phase 2 trial results suggest the possibility of a treatment that may regenerate brain cells following TBI.
Dozens of doctors and other medical professionals have been charged with exchanging opioids and other drugs for sex and cash; the first 2 patients in the United States have been treated with CRISPR; doctors have cured infants of immunodeficiency syndrome using gene therapy made from HIV.

A phase III trial evaluated intermediate-dose cytarabine plus granulocyte-colony stimulating factor (G-CSF) vs G-CSF alone prior to autoSCT in multiple myeloma.

Academic medical centers and a group representing community oncology practices have both raised concerns about CMS’ proposed reimbursement plan for chimeric antigen receptor (CAR) T-cell therapy, the individually manufactured gene treatments that are revolutionizing cancer care. The plan will be finalized next month, a year after the federal government launched a national coverage analysis to determine how to pay for these lifesaving yet expensive cancer treatments.

Patients with hematologic malignancy who are undergoing chemotherapy or a conditioning regimen for hematopoietic stem cell transplant (HSCT) are at high risk of infection because of the severity and duration of neutropenia. Fever with neutropenia is a common presentation that suggests an infection leading to empiric antibacterial therapy. To prevent infection and thus the neutropenic fever, antibacterial prophylaxis, especially with fluoroquinolones, emerged as a common practice based on results of 2 randomized controlled trials published in 2005 that showed reduced incidence of fever and bacteremia despite lack of a mortality benefit.

In this article, important concepts in the molecular testing of non–small-cell lung cancer are highlighted.

No overall survival advantage was obtained from maintenance therapy comprising nivolumab (Opdivo) alone or in combination with ipilimumab (Yervoy) over placebo in patients with extensive-stage small cell lung cancer.

Prasad S. Adusumilli, MD, sheds light on the preliminary phase I data from a trial of CAR T-cell therapy in malignant pleural disease from mesothelioma.