
The FDA cleared Century’s IND for CNTY-101 in August 2022.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The new findings are consistent with previous research, in which ophNdi1 demonstrated benefit in AMD models.

The director of the CCU/ICU at Saint John’s Health Center discussed a possible more collaborative approach to treating patients in the future.

A pivotal phase 2 clinical trial for RP-A501 is expected to initiate in Q2 2023.

The associate professor of medicine from University of Pennsylvania discussed advantages of huCART19-IL18 in NHL and CLL.

Patients treated with ALLO-715 achieved an ORR of 55.8%.

The associate attending physician at Memorial Sloan Kettering Cancer Center discussed further research that remains to be conducted with the allogeneic cell therapy.

GC012F recently showed efficacy in newly-diagnosed MM in investigator-initiated trials.

The company is developing a one-time, non-AAV2 gene replacement therapy to restore, treat, and prevent blindness of patients with RPE65 mutation-associated retinopathies.

The assistant member of the department of malignant hematology at Moffitt Cancer Center discussed updated data on the allogeneic CAR T therapy, UNICART123v1.2.

The clinical hold comes a few weeks after the company announced it was stopping enrollment in the phase 1/2 clinical trial.

Mehra and Subramanian discussed preclinical research with the investigational gene therapy SLS-004.
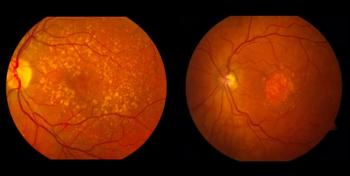
The phase 1 clinical trial is the second of Frontera’s trials to begin dosing in 2023.

Cartesian Therapeutics’ Descartes-08 will be administered as 6 weekly infusions and does not require preconditioning chemotherapy.

The professor at University Hospital Dresden discussed the positive safety profile of Unicar-T-CD123.

Decibel Therapeutics expects the trial for DB-OTO to begin in the first half of 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
The dosing of additional patients in a double-blind, placebo-controlled design will help support a BLA submission for TSHA-120.

Mesoblast is resubmitting its BLA with new CMC, survival, and mechanism of action data on the therapy and phase 3 trial.

AB-101 is also being investigated with the innate cell engager AFM13 for CD30 lymphomas.
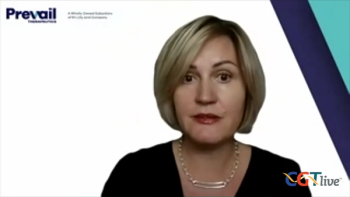
Olga Uspenskaya, MD, PhD, vice president, clinical development, Prevail Therapeutics, discussed the PROCEED trial of PR001.

Positive preclinical data related to SENTI-202 and SENTI-401 were presented at conferences last year.

MCO-010 is in phase 2 clinical trials for treating retinitis pigmentosa and Stargardt disease.

The professor of pediatrics at Stanford Cancer Institute discussed recent data from clinical trials of the lentiviral gene therapy RP-L102.

There were no deaths or safety signals identified by the DSMB for the 3 treated patients in cohort 1.

Sana Biotechnology also plans to submit another IND for the same indications for a CD22-targeted CAR T later in 2022.
No adverse events related to the infusions of stromal cells were reported.
The phase 1 portion of the trial assessing CNA3103 will start enrollment in Australia in the first half of 2023; the phase 2 portion will expand to the US.

Cilta-cel demonstrated a significant improvement in progression-free survival over standards of care.