
Steve Kanner, PhD, the chief scientific officer of Caribou Biosciences, discussed the company’s platform for genome editing.

Steve Kanner, PhD, the chief scientific officer of Caribou Biosciences, discussed the company’s platform for genome editing.

The cofounder and chief scientific officer at Encoded Therapeutics shared preclinical research with the company’s AAV and miRNA platforms.

Of 724 patients treated at the center, the cumulative incidence of secondary hematologic malignancy at 3 years posttreatment was 6.5%.

In honor of World Brain Day, observed annually on July 22 by patient and clinician communities, CGTLive is taking a closer look at this ongoing study.

The chief medical officer of Cartesian Therapeutics discussed data presented at ASGCT 2024 from a phase 2a study.

Paul Y. Song, MD, the chairman and chief executive officer of NKGen, discussed the potential of SNK01, the company’s autologous natural killer cell therapy, in treating PD.

The full 52-week analysis of the phase 2 LUNA trial are expected in the first half of 2025.

Passage Bio received the feedback on a proposal to amend the upliFT-D protocol in the context of a Type C meeting process with the FDA.

Review top news and interview highlights from the week ending July 19, 2024.
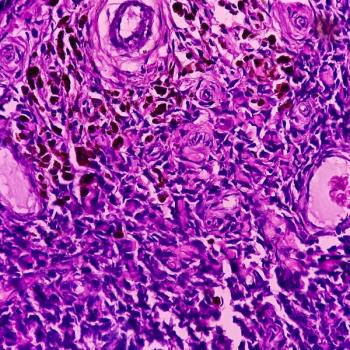
In observance of Sarcoma Awareness Month, held annually in July, we took a look back at the past year's news in cell/gene therapy for this cancer.

The associate professor of medicine at University of Colorado discussed data presented at ASCO 2024.

The gene therapy has continued to demonstrate efficacy in updated data from the phase 2 PRISM trial.

The chief scientific officer of Caribou Biosciences discussed results from preclinical research evaluating the gene editing approach.

Longeveron will present updated data from the phase 2a CLEAR MIND study at AAIC 2024 at the end of July.

The chief medical officer at Creyon Bio overviewed the development of an allele-selective TNPO2 protein for a single patient with a de novo pathology.

BRG01 is engineered to target the EBV antigen, which is frequently expressed on nasopharyngeal carcinoma tumor cells.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The FDA announced its START Pilot Program in October 2023 with a similar goal.

Manali Kamdar, MD, on The Importance of Bringing Liso-Cel to Earlier Lines of Lymphoma Treatment

Patient enrollment is anticipated to begin in the first quarter of 2025.

The senior researcher at Seattle Children’s discussed the development and validation of A2-CAR-CISC EngTreg cells.

Data from the high-dose cohort of a phase 1/2 clinical trial in South Korea will be shared in Fall 2024.

Despite the improvements, Lexeo was unable to discern a benefit on cardiovascular fitness and Peak VO2 from LX2006 with current measurements.

The medical director of clinical development at AskBio discussed setbacks in the phase 1 trial of AB-1002 gene therapy.

RG6501/OpRegen was well-tolerated, with no serious treatment-related AEs reported in follow-up data from a phase 1/2 trial.

The chief scientific officer of Caribou Biosciences discussed the company’s platform for genome editing.

Ignacio Mata, PhD, an associate professor of neurology at the Cleveland Clinic Lerner Institute, pointed out that genetic forms of PD are relatively well-understood compared to other types.

In light of the CTN clearance, Interius expects to initiate plans for a phase 1 clinical trial (INVISE) for INT2104 within the final quarter of 2024.

1x1015 vg 4D-710 has been identified as the maximum tolerated dose in the AEROW trial.

Review top news and interview highlights from the week ending July 12, 2024.