Birth control guidance provided to women taking rheumatoid arthritis therapies is lacking, and other rheumatology news reported across MJH Life Sciences™.

Birth control guidance provided to women taking rheumatoid arthritis therapies is lacking, and other rheumatology news reported across MJH Life Sciences™.

Idecabtagene vicleucel elicited a median overall survival of 24.8 months with a 51% event-free rate at 24 months in patients with heavily pretreated, relapsed/refractory multiple myeloma.

According to researchers, the findings suggest that receiving ibrutinib improves the activity of anti-CD19-CAR T cells.

With more than 3000 gene therapies in development, payers will have to grapple with the challenges of paying for these innovative but expensive therapies.

The FDA has granted a fast track designation to the CAR T-cell product AIC100 for the treatment of patients with anaplastic thyroid cancer and refractory poorly differentiated thyroid cancer.

An anticipated biologics license application for the tumor-infiltrating lymphocyte therapy lifileucel will be delayed until 2022, based on feedback from the FDA.

SRP-9001 manufactured with commercial materials demonstrated robust transduction efficiency and high gene expression levels for DMD.

Maria A. Croyle, PhD, discusses the manufacturing challenges facing the gene therapy space and her inspiration for developing a novel preservation method for viral vector-based therapies.
An off-the-shelf cellular therapy that combines Epstein Barr Virus–Specific T cells and a CD30-targeting chimeric antigen receptor product demonstrated safety and early efficacy in a group of patients with CD30-positive lymphomas.
CAR natural killer T cells that co-express GD2 and interleukin-15 were found to be safe and to demonstrate evidence of in vivo expansion and localization to metastatic sites in patients with stage IV relapsed/refractory neuroblastoma.

Locanabio CEO James Burns, PhD, shares his outlook on the quickly changing landscape of gene therapy research.
An off-the-shelf, allogeneic CD30-CAR Epstein Barr virus–specific T-cell therapy has demonstrated favorable safety and encouraging clinical activity, even when given at lower dose levels, in patients with relapsed/refractory CD30-positive lymphoma.

Although the XIRIUS study did not meet its primary end point, positive trends were observed across several clinically relevant prespecified secondary end points.
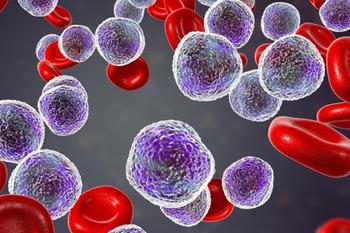
A second treatment with CD19-directed CAR T-cell therapy plus ibrutinib (Imbruvica) following failed prior CD19-directed CAR T-cell therapy and salvage treatment with ibrutinib led to greater efficiency and anti-CD19 CAR T-cell amplification, but also higher grades of cytokine release syndrome and more serious hematologic toxicity in patients with refractory B-cell non-Hodgkin lymphoma.

Preliminary results have shown that AGTC-401 and AGTC-402 seem safe and well tolerated in patients with ACHM.

Amitkumar Mehta, MD, discusses the safety profile of liso-cel and how it is advancing the relapsed/refractory large B-cell lymphoma treatment paradigm.

FIX activity was similar in participants without and with pre-existing neutralizing antibodies.
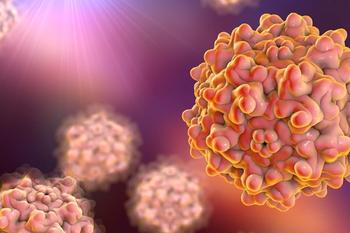
Participants in both the low- and high-dose cohorts showed improved functional outcomes at 1-year post-dose.
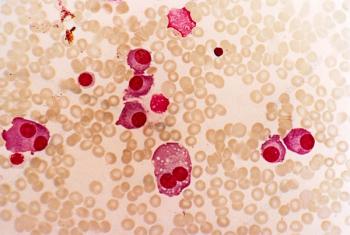
UCARTCS1A is the first allogeneic CAR T-cell product developed to target CS1 and SLAMF7, both of which are highly and consistently expressed in multiple myeloma.
The 4-day annual meeting of ISPOR—The Professional Society for Health Economics and Outcomes Research—will cover a wide range of topics. Here are 5 themes to keep an eye out for.

The low-dose cohort of RP-A501 conferred cardiac LAMP2B transgene expression.
The ongoing, phase 1/2 PrE0404 trial is evaluating the combination of ixazomib and ibrutinib in patients with relapsed/refractory mantle cell lymphoma with the goal of improving upon single-agent BTK inhibitor therapy in this patient population.

No adverse effects on weight, general activity, or survival in knockout mice and wild type littermates were observed throughout the study.

The CEO of Locanabio shares details of 2 presentations the company is making this week at the 24th Annual Meeting of the American Society of Gene & Cell Therapy.

Following 2 unexpected serious adverse events, the trial was put on clinical hold as of February 2021.

The partnership will allow for the development of disease-specific AAV vectors that will serve Biogen's current gene therapy pipeline.

The therapy was generally well-tolerated and offered substantial pain relief in patients.

Typically treated with stem cell transplantation, this lentiviral vector-based gene therapy approach may resolve challenges related to donor availability and post-transplantation complications.
MB-106, a CD20-targeted CAR T-cell therapy that has shown promise in the treatment of B-cell non-Hodgkin lymphoma, is now being considered for patients with relapsed or refractory CD20-positive chronic lymphocytic leukemia.

A new study presented at ASGCT found that patients with indications of hemostatic efficacy experienced few adverse effects with gene therapy treatment BAY 2599023.