
The chief technology and chief executive officer of Mustang Bio discussed the company’s integrated approach to drug development.

The chief technology and chief executive officer of Mustang Bio discussed the company’s integrated approach to drug development.

Experts discussed their recommendations for second-line treatment options for patients with DLBCL.

The co-founder, president, and chief executive officer of Solid Biosciences, whose own son has DMD, discussed challenges in developing gene therapies for DMD.
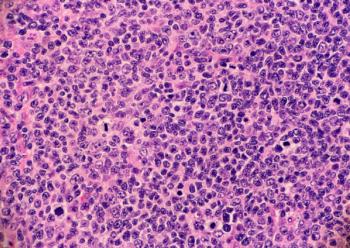
Stephen J. Schuster, MD, discussed tisagenlecleucel's efficacy and safety over other available treatments for relapsed/refractory FL.
The chief executive officer and co-founder of Flexion Therapeutics discussed the company’s pain-focused pipeline, which includes a gene therapy that targets inflammation.
A drop in endogenous Factor VIII expression was observed from treatment to 5-year follow-up despite continued demonstration of efficacy.
Joseph Sullivan, MD, and Elaine C. Wirrell, MD, discuss new developments for the treatment of Dravet syndrome beyond seizure control including gene editing and therapy.

The chief technology officer of Mustang Bio discussed the company’s approach and future plans in CAR T production.

Encouraging results from the phase 1 BrainChild-01 trial were recently published.
Heterogeneity in the cellular and molecular features of CAR T-cell products contributes to variation in efficacy and toxicity follow treatment with axicabtagene ciloleucel.
huCART19 is designed to yield longer remission rates for pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia.

The hematologist/oncologist from the Harold C. Simmons Comprehensive Cancer Center discussed the updated results of the KarMMa trial in relapsed/refractory multiple myeloma.
MCO-010 is designed to deliver multi-characteristic opsin to retinal cells.
Manuel Litchman, MD, the president, chief executive officer, and director of Mustang Bio discussed the company’s lead and second program.

The professor of medicine at the UC Davis Comprehensive Cancer Center discussed Orca-T, a high precision cell therapy, for patients with acute and chronic graft-vs-host disease.
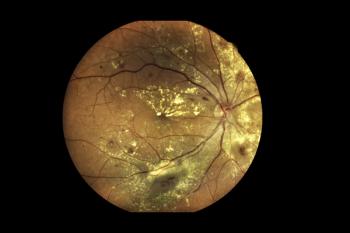
Three patients continue to show improvements in BCVA 3 years post-treatment.

The co-founder, president, and chief executive officer of Solid Biosciences, whose own son has DMD, discussed some challenges the company is facing.

The physician from the Dana-Farber Cancer Institute discussed the uptake of novel therapies in heavily pretreated multiple myeloma.

The phase 1b KEYNOTE-B79 trial of CYAD-101 for mCRC is expected to initiate in Q4 2021.

The president, chief executive officer, and director of Mustang Bio discussed the company’s pipeline.

The physician at the Dana-Farber Cancer Institute and instructor in medicine at Harvard Medical School discussed future research with CAR T-cell therapy in multiple myeloma.

Findings from an ongoing phase 1 trial were presented at the 2021 ASGCT meeting.

The founder and chief executive officer of SQZ Biotech discussed the potential of their APC platform to treat a variety of tumors.

The physician at the Dana-Farber Cancer Institute and instructor in medicine at Harvard Medical School discussed reasons to evaluate cellular therapy in multiple myeloma.
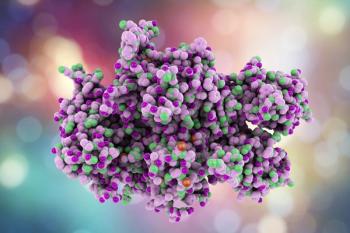
Over 90% participants had an annualized bleeding rate of 0 or a lower bleed rate than baseline after week 4 after treatment.

The hematologist from Mayo Clinic discussed the integration of CAR T-cell therapy into the treatment paradigm for heavily pretreated multiple myeloma.

James Hoffman, MD, leads a discussion about recently approved therapies and exciting future treatment options for relapsed/refractory multiple myeloma.
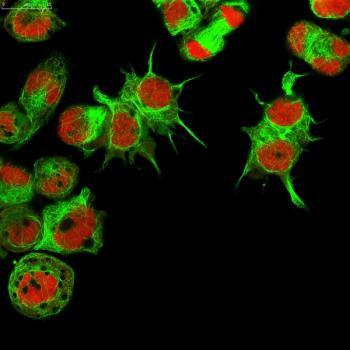
Early safety and efficacy data support the use of natural killer T (NKT) cells in patients with stage IV relapsed/refractory neuroblastoma.

The director of the Mario Lemieux Center for Blood Cancers at UPMC Hillman Cancer Center discussed strategies to manage AEs associated with CAR T therapy.

The hematologic oncologist at the Levine Cancer Institute discussed the clinical implications of tisagenlecleucel in the treatment of patients with B-cell acute lymphoblastic leukemia.