Patient-reported outcomes, 6-minute walk test, and NSAA scores all showed improvements over natural history data.

Patient-reported outcomes, 6-minute walk test, and NSAA scores all showed improvements over natural history data.
Overall response rate was 95%, including 75% of patients who achieved a complete response (CR) or better, with a median follow-up of 5.8 months at data cutoff.

Experts discuss the safety profile and cost of gene therapy for the management of sickle cell disease.
Sonny Hsiao, PhD, chief executive officer, president and cofounder, Acepodia, discussed the company’s cell therapy technologies.

The director of the Powell Gene Therapy Center at the University of Florida discussed gene therapy programs being conducted for Pompe disease.
In 2 year follow-up data, 20 patients (19.4%) remained free from high-grade recurrence.

The associate professor from the UT Southwestern Medical Center Harold C. Simmons Comprehensive Cancer Center discussed the challenges faced with CAR T-cell therapy in patients with multiple myeloma.

Review top news and interview highlights from the week ending September 24, 2021.

The recent FDA Advisory Committee meeting follows a turbulent year for gene therapy studies.

The associate professor from The University of Texas MD Anderson Cancer Center discussed mitigating CAR T-cell therapy–related cytokine release syndrome in multiple myeloma.

Eric von Hofe, PhD, president and chief operating officer of AffyImmune, discussed advantages of the Affinity tuning platform.

Brian Culley, chief executive officer, Lineage Cell Therapeutics, discussed updated data from the phase 1/2 study of OpRegen.

Jan Davidson, MD, PhD, chief medical officer, Wugen, discussed the company’s future plans and research.
The news comes after another of bluebird bio’s programs, eli-cel, was placed on clinical hold.

The professor and director of the Myeloma Center at the University of Arkansas for Medical Sciences discussed the clinical implications of CAR T-cell therapy for patients with relapsed/refractory multiple myeloma.

Oncternal and Celularity hope to produce ROR1-targeted NK, CAR-NK, and CAR T-cell therapies.
A phase 1 trial of a cell therapy for HIV is also currently underway.

The chief executive and medical officers of Cytovia Therapeutics discussed the company's therapeutic targets and cell therapy scaffold.
The CAR T-cell therapy elicited an overall response rate (ORR) of 97.9% at a median follow-up of 18 months.

The executive director of the Steele Center for Translational Medicine at the Moran Eye Center discussed the 8,000 donated eye repository that aided his research in AMD.

Patients with CDI also had more severe acute graft vs. host disease than those without CDI.

The hematologist and oncologist from the University of California, San Francisco (UCSF) Helen Diller Family Comprehensive Cancer Center discussed unmet needs with CAR T-cell therapy in multiple myeloma.

The hematologist and oncologist from University of California, San Francisco discussed the current state of cellular therapy in multiple myeloma.
The new technologies were made available as a result of the licensing agreement between MD Anderson and Bellicum Pharmaceuticals.
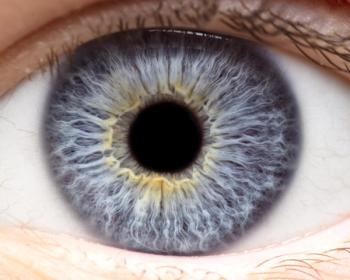
Lineage Cell Therapeutics announced promising data from interim results of the phase 1/2a clinical trial assessing the cell therapy.

The associate professor from the Harold C. Simmons Comprehensive Cancer Center of UT Southwestern Medical Center discussed the latest data from the KarMMA trial.

Review top news and interview highlights from the week ending September 17, 2021.
An interim analysis has met prespecified safety and efficacy checkpoints.
The director of the Solid Tumor Immunotherapy Lab at the University of Pennsylvania discussed his research into the mechanisms of the BET protein family and T cells.

The executive director of the Steele Center for Translational Medicine at the Moran Eye Center discussed his team’s research in the role of HTRA1.