The company stated that it has informed the FDA about its decision, which will go into effect at close of business on July 22, 2025.

The company stated that it has informed the FDA about its decision, which will go into effect at close of business on July 22, 2025.

The open-label trial, which launched on May 31, 2018, will treat up to 24 patients who have been diagnosed with ART-SCID.

The professor in the Division of Lymphoma in the Department of Hematology & Hematopoietic Cell Transplantation at City of Hope discussed unanswered questions with regard to DLBCL treatment sequencing.

The McCaw Endowed Chair of Muscular Dystrophy at University of Washington, discussed benefits and eligibility for newer DMD treatment options for older patients.

Review top news and interview highlights from the week ending July 18, 2025.
Atsena Therapeutics will advance ATSN-201 into a pivotal phase 1/2/3 trial following FDA agreement on study design and endpoints.

The CGTLive® team highlights 5 therapeutics that are nearing major decisions by the FDA.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Nathan Yozwiak, PhD, the head of research at the Gene and Cell Therapy Institute at Mass General Brigham, discussed the Institute’s efforts to bring about clinical translation of preclinical work.

According to the company, the BLA is the first for a gene-agnostic gene therapy for retinal disease to have been submitted to the FDA.

The head of research at Mass General Brigham’s Gene and Cell Therapy Institute discussed the Institute’s efforts to bring about clinical translation of preclinical work.

According to Ultragenyx, the CRL relates to a need for additional CMC information and improvements and observations from inspections of manufacturing facilities.

Derek Jackson, BS, MA, and Kilian Guse, PhD, of Pacira Biosciences, discussed the company’s symposium at ASGCT 2025.

Derek Jackson, BS, MA, the vice president of cell & gene therapy product development at Pacira BioSciences, discussed findings related to the immunogenicity of the therapy’s high-capacity adenovirus vector.

Review top news and interview highlights from the week ending July 11, 2025.

In the CRL, the FDA stated that the statutory requirement for “substantial evidence of effectiveness” was not met by the BLA and that more clinical data will be needed.
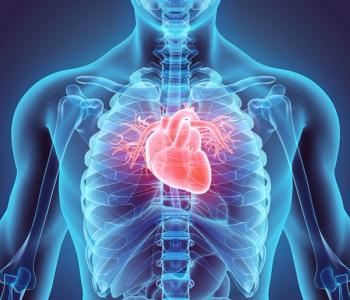
SGT-501 is intended to address CPVT by providing a full-length, codon-optimized copy of the cardiac calsequestrin gene to the muscle cells of the heart.

The McCaw Endowed Chair of Muscular Dystrophy at University of Washington, discussed how comprehensive care for DMD patients involves early diagnosis, steroid treatment, consideration of mutation-specific therapies, and more.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The vice president of cell & gene therapy product development and the vice president of genetic medicine platforms at Pacira discussed the company’s symposium at ASGCT 2025.

Jeffrey Chamberlain, PhD, McCaw Endowed Chair of Muscular Dystrophy at University of Washington, discussed how gene therapy shows promise for DMD treatment.

The vice president of cell & gene therapy product development at Pacira BioSciences discussed findings related to the immunogenicity of the therapy’s high-capacity adenovirus vector.
Alongside the new data from REGEN-007, ProKidney also announced that it will be attending a Type B meeting with the FDA later in the summer.
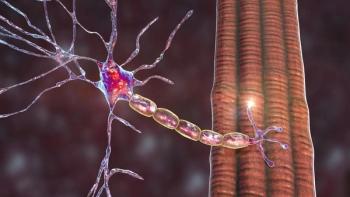
SNUG01 is intended to deliver a copy of the human TRIM72 gene to neurons via an rAAV9 vector.
The NDA is supported primarily by data from the phase 2 CT041-ST-01 randomized controlled clinical trial.

Alexey Danilov, MD, PhD, discussed the importance of reassessing sequencing stratagems in the face of a growing toolbox of targeted therapies in DLBCL.

The McCaw Endowed Chair of Muscular Dystrophy at University of Washington discusssed how gene therapy shows promise for DMD treatment, though challenges remain with delivery efficiency and determining which patients will benefit most.
Several experts weighed in on the various strategies being explored to tackle solid tumors with cell therapy, expressing optimism despite the plethora of obstacles that remain.

Review top news and interview highlights from the week ending July 4, 2025.

The FDA made the move with the view that the REMS are no longer necessary to ensure a favorable risk-benefit-ratio for the products and that dropping the requirement may increase accessibility to these therapies.