
The chief medical officer of Trisalus Life Sciences discussed the company’s PEDD system and SD-101.

The chief medical officer of Trisalus Life Sciences discussed the company’s PEDD system and SD-101.
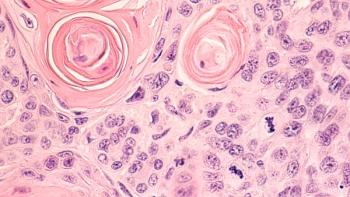
Kiromic BioPharma expects to initiate a phase 1 clinical trial for Deltacel within Q2 2023.

Updated 52-week follow-up data were presented at the EBMT meeting.

The cofounder and chief scientific officer of Xcell Biosciences discussed the limitations of the methods currently used for modeling in preclinical cell therapy research for solid tumors.
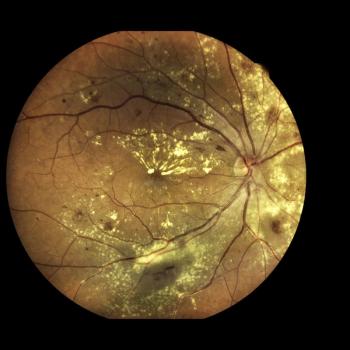
New data from imaging analyses on RG6501 were presented at the ARVO 2023 Annual Meeting.

The study found significant improvements in BCVA, in contrast to several other studies evaluating Luxturna.

The data, presented at ARVO's 2023 conference, also showed a favorable safety profile.

Review top news and interview highlights from the week ending April 28, 2023.
The chief scientific officer of Aurion Biotech discussed the properties of the cell therapy for corneal dystrophies, which was recently approved in Japan.


The new data builds upon positive long-term follow-up data from clinical trials.

The head of the Department of Ophthalmology at the Center for Medical Genetics, Ghent University Hospital, discussed the results of a post-marketing study presented at ARVO’s 2023 conference.

The scientific cofounder and chief executive officer of Alloplex Biotherapeutics discussed early data from a phase 1 trial of the PBMC-derived therapy.

The safety profile of lenadogene nolparvovec at 5-years post treatment was similar to 2-year data.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The associate director of research at Lyell Immunopharma discussed the company’s programs and newest technologies aimed at solid tumors.

The instructor at Stanford Institutes of Medicine discussed the key takeaways of her research into the factors that affect CAR-T expansion.

No serious TEAEs have been related to ATSN-101 with data up to 6 months after treatment.
The chief scientific officer of Aurion Biotech discussed the investigational cell therapy AURN001.

STZ eyetrial’s treatment rAAV8.hPDE6A was otherwise deemed well-tolerated.

The BLA submission comes after a series of delays which have pushed lovo-cel behind Vertex’s exa-cel in the race to approval.

The commercially available gene therapy, developed by Spark Therapeutics, was evaluated in multiple studies presented at ARVO’s 2023 conference.

The new positive data on the AAV based gene therapy builds upon real-world data presented at ARVO’s conference in 2022.

The research scientist at Moffitt Cancer Center discussed the results of the preclinical study she presented at the 2023 AACR annual meeting and avenues for further research.
JadiCell is already being evaluated for the treatment of COVID-19 associated ARDS in a phase 3 trial.

The professor of breast cancer research at Indiana University discussed new research with the TONSL gene from Indiana University.

Review top news and interview highlights from the week ending April 21, 2023.

Overall, 6 patients were treated in a feasibility study for a disease control rate of 33.3%.

The research scientist at Moffitt Cancer Center shared the rationale behind the preclinical study she presented at the 2023 AACR annual meeting.

The pediatric clinical pharmacist in stem cell transplant and cellular therapy at the University of Minnesota Masonic Children’s Hospital and M Health Fairview offered an in-depth preview of the upcoming virtual meeting.