
The professor from The University of Texas MD Anderson Cancer Center discussed long-term follow-up analysis of the phase 2 ZUMA-5 trial.

The professor from The University of Texas MD Anderson Cancer Center discussed long-term follow-up analysis of the phase 2 ZUMA-5 trial.
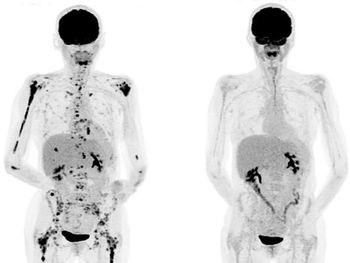
PET/CT plays a central role in evaluating response after CAR T-cell therapy.

Improved knowledge of the effects of mutations in the EYS gene may help inform new treatments.

Review top news and interview highlights from the week ending December 17, 2021.

Safety and the recommended dose of CYAD-211 and the lymphodepletion regimen served as the primary end point of the study.
Patients treated with cilta-cel had a 76% reduction in the risk of death compared to patients treated with physician's choice of treatment.

The vice chair, Blood and Marrow Transplant and Cellular Immunotherapy Program, and co-leader, Immuno-Oncology, Moffitt Cancer Center, discussed the results of the phase 3 ZUMA-7 trial.

AJ Joshi, MD, chief medical officer, Atara Biotherapeutics, discussed safety findings from the phase 3 ALLELE study.

Annualized bleeding rate, spontaneous bleeding, and joint bleeding rates were all reduced in patients dosed with fitusiran compared to those receiving on-demand treatment.
Over half of patients in the fitusiran arm of the ATLAS-INH study had 0 treated bleeding events.

Following GS030 optogenetic therapy, the first treated patient was able to locate and count objects on a table and could identify crosswalks in the street.
The findings, which were simultaneously reported in the New England Journal of Medicine, support beti-cel as a potentially curative, one-time treatment option for these patients.
ADI-001 does not require genetic engineering to remove TCRs to avoid GvHD, making them ideal for an off-the-shelf cell therapy.

The hematologists from Moffitt Cancer Center and University of Texas MD Anderson Cancer Center discussed navigating the referral process for CAR T-cell therapy in hematologic malignancies.
Patients treated with the CAR T-cell therapy had an over 4-fold increase in event-free survival.

Review top news and interview highlights from the week ending December 9, 2021.
All evaluable treated participants experienced a reduction in vaso-occlusive events after treatment with ARU-1801.

CSL Behring plans to submit regulatory applications in the US and EU in the first half of 2022 for the AAV gene therapy etranacogene dezaparvovec.

The hematologist/oncologist from Mayo Clinic discussed the role of HLA loss in common cancers and its use as a biomarker.
Cedrik Britten, MD, chief medical officer, Immatics discussed data on IMA203 presented at SITC 2021.
Autologous adipose stem cell data in a small cohort provides a feasibility for future validation studies.
The hematologists from Moffitt Cancer Center and MD Anderson discussed managing CAR T-cell therapy-associated toxicities.
Current studies are using a polygenic risk score to evaluate the risk and clinical outcomes in primary open-angle glaucoma (POAG).
Adicet Bio reported positive interim data from the phase 1 GLEAN study.

Scott Requadt, chief executive officer, Talaris Therapeutics, discussed the company’s goal to reprogram the immune system.

The companies will combine Imugene’s CF33-CD19 oncolytic virus and Celularity’s investigational placental-derived CAR T-cell therapy.
Findings from the phase 2 INFINITY trial identified dose-dependent safety outcomes of ADVM-022 in patients with diabetic macular edema.
Further understanding of how COVID-19 vaccinations affect CAR T-cell therapy recipients is needed.
Ralph Laufer, PhD, chief scientific officer, Lysogene, discussed LYS-SAF302, its mechanism of action, trial data, and further research.
Julio Chavez, MD, MD, discussed treatment options for LBCL after relapsing on CAR T-cell therapy.