
Clinical and industry leaders share their perspectives on the importance of collaboration in developing treatments for rare diseases.

Clinical and industry leaders share their perspectives on the importance of collaboration in developing treatments for rare diseases.

The president and chief operating officer of Rocket Pharmaceuticals discussed the importance of Rare Disease Day.

The phase 1b KEYNOTE-B79 trial is investigating the allogeneic cell therapy in participants with metastatic colorectal cancer.

The director of the Powell Gene Therapy Center at the University of Florida discussed the importance of Rare Disease Day.

Crystal Proud, MD, outlines advances in technology and research in neuromuscular diseases.
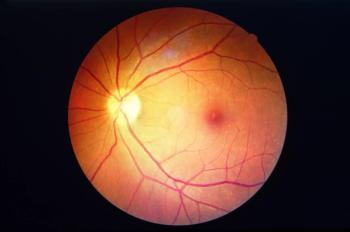
The professor of ophthalmology from Radboud University, The Netherlands, discussed ongoing gene therapy trials in Stargardt disease.

Review top news and interview highlights from the week ending February 25, 2022.
An interim safety review of 81 patients informed the DSMB's recommendation for the trial to continue.

The chief scientific officer and senior vice president of the Parkinson’s Foundation discussed the organization’s initiative to advance genetic testing.
Cory Nicholas, PhD, cofounder and chief executive officer, Neurona Therapeutics, discussed the company’s lead program in mesial temporal lobe epilepsy.

The professor and head of coagulation disorders and Comprehensive Care Centre, University Hospital of Frankfurt, Germany, discussed new efficacy findings from the HOPE-B study.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The director of the hemostasis and thrombosis program at Children’s Hospital Los Angeles discussed mitigation strategies in trials and clinic.

The trial design was informed by both patient and physician perspectives.

The director of the Pediatric Hemophilia and Coagulation Disorders Program at CS Mott Children’s Hospital discussed the advantages of AAV gene therapies.

The pause follows a fatal serious adverse event in the first patient treated in the highest dose cohort.
The trial, sponsored by Mustang Bio, will continue to enroll patients and doses will be escalated to 300 million cells.

The pheNIX clinical hold follows an announcement that BioMarin’s PKU hold may last several quarters.
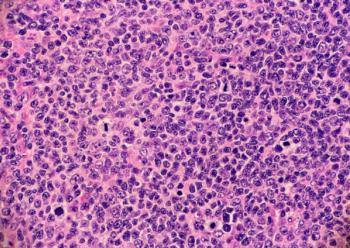
The new application is based on data from the phase 3 TRANSFORM trial.
The therapy has been well-tolerated in the 15 patients treated so far.

The chief scientific officer of FibroBiologics discussed the benefits of fibroblast cell technology in MS.
Researchers will use the model to study how lack of pigmentation affects RPE physiology and function.

The FDA cleared SBT-101's investigational new drug application earlier in February 2022.

The ophthalmologist from John A. Moran Eye Center discussed the close association between disease progression and uncovered risk alleles.

Review top news and interview highlights from the week ending February 18, 2022.

The company also announced that the phase 3 study of val-rox has completed enrollment.

Matthew Gantz, president and chief executive officer, Castle Creek Biosciences, discussed FCX-013 for the potential treatment of scleroderma.

Interim data from the SPOTLIGHT study were presented at the ASCO GU Symposium.

Myrtelle’s therapy targets oligodendrocytes to deliver a functional copy of the ASPA gene.